You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The ADAA has an obligation to disseminate knowledge in the field of dentistry. Sponsorship of a continuing education program by the ADAA does not necessarily imply endorsement of a particular philosophy, product, or technique.
Introduction to Oral Cancer Genetics
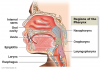
Oral cancer can develop in any part of the oral cavity or oral pharynx (Figure 1), but the most common sites are the floor of the mouth, lips, soft palate, and tongue. Mouth cancer is considered anything from the last molar forward to the lips, including the part of the tongue seen in the mirror, the hard palate and the inside of the cheeks. Anything behind the last molar is a different area called the oropharynx, which includes the tonsils, soft palate and the base of the tongue. Cancers of the hard palate are not common in the United States. The most recent estimates in 2013 from the American Cancer Society show that each year over 36,000 people will be diagnosed with oral or oropharyngeal cancer and over 6,850 people will die from these cancers. In 2017, approximately 49,750 people in the United States will be newly diagnosed with oral cancer. For more than a decade there has been an increase in the rate of occurrence of oral and oropharyngeal cancers. While many view oral cancer a rare cancer, mouth cancers will be newly diagnosed in approximately 132 new individuals each day in the United States alone, and one person dies from oral cancer every hour of every day. Add in the sub category of laryngeal throat cancers, and the rates of occurrence and death are considerably higher at about 12,000 additional new cases annually.
Oral cancer is more common than cancers of the brain, liver, bone, stomach, cervix and ovaries; it is even more common than leukemia. When oral cancer is detected early, the cure rate can be as high as 80-90% depending on the location of the cancer. Unfortunately, by the time a medical or dental diagnosis is made, about two-thirds of oral cancers are advanced lesions with evidence of invasion and metastasis. This leads to high morbidity and mortality in spite of advances in surgical techniques, radiotherapy and chemotherapy. Little has changed in the last 50 years and approximately half of the people who develop oral cancer die because of the disease. Oral cancer is particularly dangerous because it has a high chance of producing secondary tumors, usually in the lymph nodes of the neck. This increased risk factor can last up to a decade after the first episode of tumor invasion.
Annually, oral cancer kills more people than female cervical cancer and costs society more than $2 billion in treatment costs and lost wages. Many of these malignant conditions could be arrested if detected earlier. The dental profession has a unique opportunity during patient assessment to detect oral cancer while it is still asymptomatic, innocuous, and unsuspected. It is estimated that 5-10% of routine dental patients have some unusual findings, from something as harmless as mandibular or maxillary tori to something as serious as a malignant neoplasm. Oral squamous cell carcinoma accounts for 90% of all primary oral malignancies. It is possible for the oral cavity to be the site for tumor metastasis from other locations, such as the breast, lung, and gastrointestinal tract.
Cancer of the three major salivary glands (parotid, submaxillary, and sublingual) is considered a separate form of oral cancer, as are cancers of the jaw and the muscles of the face. Therefore, it is important to identify the area the cancer originated from, even if it has spread to other areas of the head. The origin is the factor that determines the type of cancer.
There are two dissimilar pathways by which most people develop oral cancer. One is through the use of tobacco and alcohol, a chronic historic problem and cause. The other pathway is through exposure to the HPV-16 virus, a recently identified etiology, and the same one which is responsible for the vast majority of cervical cancers in women today. Less than 7 percent of the individuals who do get oral cancers are unable to trace an identified cause – it just occurs and is believed that these are likely related to some genetic predisposition. Unfortunately at this time, the majority of oral cancers are found as late stage cancers, and this explains for the very high death rate of about 43% at five years from diagnosis for all stages and etiologies combined at time of diagnosis, and high treatment related morbidity in survivors. Late stage diagnosis is not occurring because most of these cancers are difficult to discover. Some HPV – origin disease cancers have unique discovery issues but it is because of a lack of public awareness together with the lack of a national program for preventive screenings which would yield early discovery by medical and dental professionals. Worldwide the problem is far greater, with new cases annually exceeding 640,000 cases.
Risk Factors
Oral cancer affects men twice as often as women. It is more common among African-Americans than Caucasians, and seen more often in those of lower socioeconomic status. The average age at onset is 62 years old. Most patients are over 40 years old, but it is also important to remember that oral cancer can strike at any time and anyone. The exact causes for those affected at a younger age are not known at this time. There are some probable links to young males who use chewing tobacco. Other researchers believe that the underlying link is viral based, given that the amount of time these individuals have been exposed to known causative agents, such as tobacco, is short.
Gender
From a gender perspective, oral cancer had affected six males for every female for decades. The ratio is now two males for every one female. Published studies do not draw concrete conclusions as to why females have closed the gap, but many believe that lifestyle changes in which the number of women smokers has increased over the last twenty years plays a significant role. Men are twice more likely to get oral cancer than women and the American Cancer Society attributes this gender risk to higher rates of alcohol and tobacco use by men; however, more men of a younger age are being diagnosed with HPV-related forms of oral cancer.
Ethnicity
From a racial and an ethnic perspective, oral cancer occurs twice as frequently in the African American population as it does in the Caucasian population. Among African American men, the oral cavity is the fourth most frequent site of cancer. Survival statistics for an African American individual over a five-year period are about 40%, while for a Caucasian individual it is approximately 60%. Researchers are unlikely to find a genetic reason for this anytime soon. Published statistics do not take into consideration socio-economic factors such as availability of proper health and dental care, education, income levels, and the increased use of both tobacco and alcohol by different ethnic populations.
Age
Age is frequently named as a risk factor for oral cancer since most cases occur in those over the age of 40. The average age of those diagnosed with oral cancer is 62. The ages of the diagnosed patients may indicate a time component in the biochemical or biophysical processes of aging cells that allows malignant transformation. New trends inevitably emerge, and recently there has been an increase in the number of patients in their 20's and 30's who have developed oral cancer, especially cancer of the tongue, without any apparent risk factors such as tobacco and alcohol use or immunosuppression.
Tobacco
Oral cancer is a multi-step process of accumulated genetic mutations caused by a combination of diet, alcohol and tobacco use, oral hygiene, lifestyle, lowered immunity, and genetic susceptibility. The use of tobacco in all of its forms (cigarettes, cigars, pipes, marijuana, and chewing tobacco) is a well-established risk factor for not only oral cancer, but other cancers as well. In oral cancer, it is considered the number one risk factor. Even children who use chewing tobacco, often to emulate famous baseball players, have developed oral cancer. A strong association has been noted between development of oral premalignancy in the form of erythroplakias and the use of chewing tobacco combined with alcohol consumption. Pipe smokers have a greater incidence of developing lip cancer.
The majority of carcinogens in tobacco smoke are the byproduct of pyrolysis. In pipe and cigar smoke, these byproducts are often found in much higher concentrations. Chemical analysis reveals that smoke from a single cigarette is composed of over 4,000 different components, including some that are pharmacologically active, carcinogenic, mutagenic or toxic. Smokeless tobacco contains double the amount of nicotine as in an average cigarette. Besides increasing the risk of mouth cancer by up to four times, it creates problems for the heart by tightening blood vessels and raising the blood pressure. One tin of chewing tobacco can release as much nicotine into the body as smoking 60 cigarettes.
Smokeless tobacco which is manufactured through aging, curing, and fermentation, also contains carcinogens, some at extremely high levels. Tobacco-specific N-nitrosamines (TSNAs) form from nicotine and other tobacco alkaloids. During tobacco chewing and snuff dipping, it is likely that additional amounts of carcinogenic TSNAs are also formed deep in the tissues in the oral cavity. In relationship to the enormous number of toxic and carcinogenic compounds that exist in tobacco and tobacco smoke and the level of exposure to these agents among tobacco users, it is not unexpected that tobacco use is so strongly implicated in the causation of human cancer. A number of these compounds have been directly implicated in the production of oral carcinomas and exist in both cigarette smoke and in smokeless tobacco products in amounts that have generated oral malignancies in laboratory animals.
The more tobacco that is used for a longer period of time, the higher the risk for oral cancer. According to the Oral Cancer Foundation, numerous studies examining the relative risk for oral cancer among former smokers have found that the risk for oral cancer was lower among former smokers after the first few years of abstinence than for those who continued to smoke or use some form of tobacco. The studies found that the oral cancer risk decreased by approximately 50% after 3 to 5 years of smoking abstinence.
The risk declines to almost normal over a 10-20 year period of smoking abstinence.
Alcohol
Excessive alcohol use also can increase the risk of developing oral cancer. Beer, hard liquor, and wine have been associated with oral cancer, although hard liquor and beer have an elevated correlated risk. Studies that have found alcohol use to be a factor for oral carcinogenesis have usually concluded that the level of consumption was significant.
One theory suggests that alcohol generates metabolites that are carcinogenic to humans; the major metabolite of ethanol is acetaldehyde, a known carcinogen. Acetaldehyde may be produced both systemically and by the oral microflora. Alcohol may also act as a solvent for tobacco, making it easier for the carcinogens to infiltrate the oral mucosa. The use of both alcohol and tobacco simultaneously generates a greater risk for oral cancer than using either substance alone. It is estimated that the combination of both smoking and drinking causes approximately 75% of all oral and pharyngeal cancers in the United States. As with tobacco, lowering the frequency of alcohol usage gradually lowers the risk of cancer to that of non-drinkers who don’t smoke.
Diet
Although dietary factors have been identified as having a possible association with oral cancer, accumulated scientific evidence that use of tobacco and alcohol increases oral cancer risk far outweighs any evidence linking a deficient diet to increased risk.
Research suggests that diets lacking fruit and vegetables could contribute to oral cancer, as well as other forms of cancer. Fruits and vegetables contain beneficial antioxidants that trap harmful molecules called free radicals. Antioxidants can help prevent cancer causing genetic mutations. Natural carotenoid compounds, dietary selenium, folate, and vitamins A, C, & E reportedly offer protection against cancer development. Low beta-carotene ingestion has been linked with an increased risk of lung, laryngeal, gastric, ovarian, breast, cervical, and oral cancers. It has also been shown that a low intake of fruits and vegetables, which are the primary sources of beta-carotene, is also related to a universal increased cancer risk and mortality. On the other hand, an increased consumption of fruits and vegetables has been connected with a decreased risk of oral or oropharyngeal cancer when compared with low intake levels. A low intake of vitamin C has been linked with an increased risk of cancers of the stomach, esophagus, oral cavity, larynx, and cervix. Patients who consume high levels of vitamin C and fiber have half the risk of oral cancer as those with the lowest level of consumption. Patients with low serum levels of vitamin E have more than double the general risk of gastrointestinal cancers. The use of vitamin E supplements is associated with a diminished risk for oral and pharyngeal cancer. Consumption of Cantonese salted fish from early childhood on into adulthood has been associated with oral cancer formation in some Asian countries. Betel nut chewing in Indian populations is strongly associated with tooth loss and oral cancer, due to prolonged irritation of the tissues.
Up to half of all oral cancer cases are partly due to poor diet. A diet rich in fruit and vegetables will not only keep your body fit and healthy, it will help to reduce the risk of oral and other cancers. Non-starchy vegetables and fruits (not salted or pickled), and foods containing carotenoids, can actually help to reduce mouth cancer risk. Oral cancer risk is lower in people with the highest intake of the following foods:
• Fruit – 48% lower risk.
• Vegetables – 34% lower risk.
• Vitamin C supplements – 24% lower risk (versus never-users).
• Calcium supplements – 36% lower risk (versus never-users).
• Caffeinated coffee – 39% lower risk in 4 cups/day (versus non-drinkers).
• Green tea – 20% lower risk.
The most consistent dietary findings across multiple cultural settings are that high fruit consumption has a protective effect and that high alcohol consumption has a carcinogenic effect.
Oral Hygiene
Each teaspoon of saliva contains about 1 billion bacteria, which are making waste products that cling to the teeth in the form of plaque. This sticky white film can easily be removed with proper daily brushing and flossing techniques. If plaque isn't removed, it will calcify over time and cause irritation to the teeth and gums. Plaque itself is not shown to cause oral cancer, but it aids other irritants like tobacco and alcohol to stick in the mouth, irritate the tissues, and stimulate the cells to divide. The more cells that divide, the greater chance one of them will become cancerous. The common thread of many risk factors is irritation, which can lead to a lot of cell division.
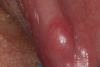
Ill fitting dental appliances such as partials and dentures irritate the gingiva and trap debris. Continued irritation can lead to lesions and eventually develop into oral cancer (Figure 2).
Lifestyle
The decision to smoke or drink is certainly a lifestyle risk factor. Those who do not smoke or drink alcohol have a lower risk of developing oral cancer than those who choose to indulge. Ultraviolet radiation is another lifestyle risk. Cancer of the lip is one oral cancer that has declined over the past few decades, most likely due to society's increased awareness of the damaging effects of prolonged exposure to sunlight and the increased use of sunscreen products.
Lowered Immunity
Infections such as syphilis can lead to cancer over time due to poor healing mouth sores that are repeatedly irritated. The constant attempt to heal affected tissue leads to chronic cell division and a greater chance for cancer to develop. Lowered immunity from AIDS or transplant anti-rejection drugs will increase the risk for many cancers, including those of the aero-digestive tract (the area from the nose and mouth to the lungs and stomach).
Environmental
Environmental tobacco smoke, also known as second hand smoke, has been named a plausible cause of mouth cancer. Mouth cancer risk is 87% higher in those who have never smoked and that have been exposed to tobacco smoke at home or work, compared with unexposed non-smokers. Studies have also shown that the risk of mouth cancer is more than twice as high in people who have never smoked exposed to second-hand smoke at home or work for 15 years or more, compared with unexposed never-smokers.
Human Papilloma Virus
The human papilloma virus (HPV) is a double-stranded DNA virus that infects the epithelial cells of skin and mucosa. The moist epithelial surfaces include all areas covered by skin or mucosa such as the inside of the mouth, throat, tongue, tonsils, vagina, cervix, vulva, penis, opening to the urethra and anus. Transmission of the virus occurs when these areas come into contact with a virus, allowing it to transfer between normal epithelial cells and infected epithelial cells. While it is recognized now that both conventional and oral sexual contact are means of transferring the HPV virus through direct skin to skin contact, it is still inadequately understood what other transfer pathways may exist and pose a threat. It is highly unlikely that the virus can live for long on inanimate objects outside of a cell or moist environment.
HPV has long been associated with cancers in the anogenital region, but in 2007 was also recognized as a cause of oropharyngeal cancer. The leading cause of oropharyngeal cancer is from HPV; a very small number of oral cavity cancers also occur from HPV. The HPV family contains almost 200 strains, and it is one of the most common viruses found in the United States population. It is important to understand that of these 200+ strains, only nine are associated with cancers affecting humans. Of the nine that are high risk, only one is closely associated with oropharyngeal cancer, the HPV16 strain. Several more are associated with benign growths and the vast majority we have no evidence, other than they exist, that they harm us in any way.
According to the Centers for Disease Control and Prevention (CDC), 80% of Americans will have HPV infections at some point in their lifetime and 99% will clear these infections without result, or even knowing that they had the infection, as it produces no noticeable symptoms. The body’s immune system is usually able to get rid of many infections, including the HPV infection. Oropharyngeal cancers continue to still be a very small risk to most of us in the United States even though increasing rapidly in frequency. An informed patient will be able to self-discover something which doesn’t seem right and bring it to the attention of healthcare professionals for the earliest possible diagnosis of a problem. Early discovery has many positive impacts relating to cancer with potentially longer life spans, and reduction of treatment related morbidity, significantly improving post cancer treatment quality of life for many.
More than 40 HPV types can be easily spread through direct sexual contact, from the skin and mucous membranes of infected people to the skin and mucous membranes of their partners. They can be spread through various modes of sexual contact. Other types of HPV are responsible for non-genital warts and are not sexually transmitted. Sexually transmitted HPV types fall into two categories – low–risk HPVs and high–risk HPVs. Low–risk HPVs do not cause cancer but can cause skin warts known as condylomata acuminate on or around the genitals and anus. HPV6 and HPV11 cause 90% of all genital warts. Both strains also cause recurrent respiratory papillomatosis, a less common disease in which benign tumors grow in the air passages leading from the nose and mouth into the lungs. High–risk HPVs can cause cancer. Roughly a dozen high-risk types have been identified and two of these are responsible for most HPV–caused cancers: HPV16 and HPV18.
Today, HPV infections are the most common sexually transmitted infections in the United States with approximately 14 million new genital HPV infections occurring annually. The CDC estimates that more than 90% of sexually active males and more than 80% of sexually active females will be infected with at least one type of HPV at some point during their lifetime. Approximately 50% of these infections are with a high–risk HPV strain.
Most high–risk HPV infections occur without any symptoms, resolve themselves without treatment within 1 to 2 years, and do not lead to cancer. Other HPV infections, however, can continue for many years, leading to cell changes that, if untreated, may progress to cancer.
High–risk HPVs are the causative agents in a number of cancers. Worldwide, they account for 5% of all cancers. In the United States, they cause approximately 3% of all cancers in women and approximately 2% of all cancers among men. Nearly all cases of cervical cancer are caused by HPV, and just two HPV types, 16 and 18, are responsible for virtually 70% of all cases. About 95% of anal cancers are caused by HPV with most of these caused by HPV type 16. Almost 70% of oropharyngeal cancers are caused by HPV and in the United States, more than half of cancers diagnosed in the oropharynx are linked to HPV type 16. HPV is the causative agent in about 65% of vaginal cancers, 50% of vulvar cancers, and 35% of penile cancers. The majority of these are caused by HPV type 16.
HPV infects epithelial cells which are organized in layers, cover the inside and outside surfaces of the body. Once HPV enters an epithelial cell, the virus begins to make the proteins it encodes. Two of the proteins made by high – risk HPVs (E6 and E7) interfere with cell functions that normally prevent excessive growth, helping the cell to grow in an uncontrolled manner and to avoid cell death. Many times these infected cells are recognized by the immune system and eliminated. Sometimes, however, these infected cells are not destroyed, and a persistent infection results. As the persistently infected cells continue to grow, they may develop mutations in cellular genes that promote even more abnormal cell growth, leading to the formation of an area of precancerous cells and, ultimately, a cancerous tumor. Other factors may increase the risk that an infection with a high–risk HPV strain will succeed and potentially develop into cancer. These risk factors include:
• Smoking (cigarettes or pipe) or chewing tobacco (increases risk of oropharyngeal cancer)
• Having a weakened or stressed immune system
• Having had multiple children (increases risk of cervical cancer)
• Long–term use of oral contraceptive (increases risk of cervical cancer)
• Poor oral hygiene (increases risk of oropharyngeal cancer)
• Chronic inflammation
Researchers believe that it can take between 10 and 30 years from the time of an initial HPV infection until a tumor forms. However, for example, even when severely abnormal cells are seen on the cervix these do not always lead to cancer.
Genetic Predisposition
An individual with a genetic predisposition for cancer is a person with an increased chance of developing cancer due to inherited genes. These inherited genes make body cells more sensitive to environmental factors, such as sunlight and tobacco, and, therefore change normal body cells into cancer cells. It is now established that up to 10% of all cancers have a strong hereditary component. Role of genetic component in the development of oral cancer is being suggested by several studies showing familial clustering. A clustering of oral cancer has been seen in certain ethnic groups, like Ashkenazi group in Israel; with incidence being double as compared to other Jewish population in that country, yet, the basis of this genetic susceptibility is not well understood, and still being studied.
Tumor Formation
Some viruses are suspected in the development of oral cancer. Viruses can get into the cells of the oral cavity and change the genes in them to form a cancer cell. This process is known as "oncogene activation." HPV, particularly the HPV-16 and the HPV-18 strains, and the Herpes Viruses are now considered contributors to some oral cancers. The HPV-16 and the HPV-18 strains are the causative agents in cervical cancer, accounting for 70% of all cervical cancers. DNA from HPV and particular herpes viruses (including the Epstein-Barr, cytomegalovirus, and herpes simplex) has been discovered in biopsies from the oral cavity. Each year, more than 2,370 women and 9,350 men are diagnosed with HPV associated oropharyngeal cancer. Studies have shown a dramatic increase of 225% when looking at cases of HPV associated oropharyngeal cancer from 1988 to 2004. As with many other types of oral cancer, oropharyngeal cancer is 2 times more likely in men than women. Genes encoded within these viruses are involved in the initiation of the multiple steps needed for a normal cell to become malignant. Two genes, Rb and p53 regulate normal cell division. Rb has the role of separating transcription factors needed for progression through the cell cycle, preventing the normal cells from dividing until it has separated enough transcription factors. Rb is a tumor suppressor gene that segregates a protein called E2F. When normal cells are infected with HPV, the E7 gene from HPV binds to Rb, releasing E2F and other proteins, signaling the start of the cell cycle. This cycle continues as long as E7 remains attached to Rb. Uncontrolled cell division is a sign of malignancy.
The other gene that HPV attacks is the p53 gene, responsible for repair of damaged DNA and apoptosis in the event that repair is impossible. In malignant cells, p53 is often nonfunctional or missing entirely. The viral E6 protein binds to p53 making it nonfunctional. Damaged DNA is then replicated continually since the non-functional or missing p53 does not initiate apoptosis. E6 protein also activates telomerase, an enzyme that synthesizes the telomere repeat sequences. The activation of this enzyme ensures the repeated cell cycle that continually produces more viral cells, leading to malignancy.
Scientists have linked a new virus (human herpes virus-8) with AIDS-related Kaposi's Sarcoma, another cancer that prefers the head and neck region. Oral lesions are present in about half of the Kaposi's Sarcoma cases, with the hard palate and the gingival areas being the most commonly affected areas. This new virus has been found in all forms of Kaposi's Sarcoma, insinuating that it might be involved in sarcoma development. A direct role has not yet been identified.
Signs and Symptoms
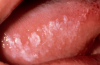
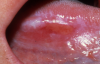
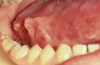
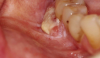
One of the greatest dangers of oral cancers, especially in the early stages, is that it can go unnoticed. Unfortunately, and all too often, the earliest symptoms are noticed when body function is affected. Early oral cancer symptoms can be painless, hidden, and not bothersome. Oral precancer and cancer exhibit a wide range of clinically detectable alterations that may range from an early, subtle change in surface texture, color, or elasticity to a more obvious lesion. Changes in the oral epithelial cells often present as white, or red/white patches called leukoplakia (Figure 3) or as velvety, red patches called erythroplakia (Figure 4). These changes represent cellular alterations that result from genomic changes within the surface epithelial cell population, including changes in DNA content, loss of heterozygosity, and genetic alterations in a cascade fashion that lead to the formation of invasive squamous cell carcinoma (Figure 5 and Figure 6).
The most common intraoral sites for squamous cell cancer are the lateral borders and ventral surfaces of the tongue, floor of the mouth, and the oropharynx. Any of the signs and symptoms that persist for longer than two weeks after removal of potentially irritating factors and/or application of therapeutic measures should be considered a cancer until proven benign by biopsy and microscopic evaluation.
Other cancers found less frequently in the oral cavity include carcinomas of salivary gland origin, lymphomas, melanoma, and bone and soft tissue sarcomas. Cancers that begin in other parts of the body may metastasize to or exhibit manifestations in the oral cavity. The most common metastases are from tumors in the lung, breast, colon, and kidney. Manifestations of leukemia, lymphomas, and multiple myeloma may also be present within the oral cavity.
(Common signs of oral cancer are listed in Table 1.)
Diagnosis
The diagnostic phase of patient management begins with an assessment of the medical history and the possible impact and overall management of any oral disease or condition. Many times patients are not even aware they have a lesion or lump in the oral cavity, especially if it is hidden beneath the tongue. These deviations are often discovered during a routine head and neck examination, whether the patient is in for their recare visit or for some restorative work. The American Dental Association (ADA) stresses the importance of doing routine head and neck exams and oral cancer evaluations on each and every patient. The screening takes just 90 seconds and can make the difference between life and death for the individual. Although there is no definitive set of steps for an oral cancer screening, there are several areas that should be routinely checked. Dental practitioners would be wise to check the following areas: sides and underside of the tongue, lips and cheek, floor of the mouth, palate, tonsils, and finally the neck.
Unfortunately, in the recent past, the number of oral cancers diagnosed at an early stage had not increased, possibly reflecting the lack of effective professional and public education. This trend is beginning to improve as more practitioners are doing regular screenings and diagnostic aids are improving. Early detection reduces morbidity and decreases mortality (Figure 7).
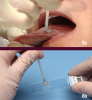
Ideally, a pathologist must test any observed suspicious mucosal abnormality obtained by using a scalpel, punch biopsy, or brush biopsy for evaluation. One essential tool for painlessly obtaining a full thickness sample of oral epithelium is the OralCDx® Brush (Figure 8 a and b). The OralCDx® is a brush biopsy followed by a computer-assisted analysis. The suspected lesion is brushed gently with the OralCDx® brush, then smeared over a glass microscope slide, fixed, and then sent to a laboratory that scans the slide. Results from the lab are usually available within 24 hours. Should positive results be returned through this system, the brush biopsy must be followed by a conventional biopsy procedure. Recent research has shown that brush biopsies such as OralCDx® result in a high number of false positive results. One researcher reports that of 152 positive results from a brush biopsy there were only 12 cases that were truly dysplastic. Scalpel and punch biopsies are used for highly suspicious lesions, whereas the OralCDx® is used for lesions of uncertainty. Early-stage oral cancers mimic many benign conditions seen more frequently. This is when the OralCDx® is best used.
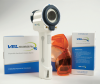
Chemoluminescent lights are also available under the name of ViziLite® and VELscope®. VELscope® (Figure 9 and Figure 10) will fluoresce differently when normal tissues and the suspected tissue is exposed to certain wavelengths of light. Precancerous tissues become "excited" under exposure to this wavelength. ViziLite® first involves washing the mouth with acetic acid followed by using blue phenothiazine dye to mark the lesions. The wavelength of the illumination is absorbed by normal tissues and reflected back by abnormal tissues, making them easier to identify.
Toulidine blue, a selective binding stain on malignant cells, is a useful supplement to a clinical examination and biopsy. Use of this stain is economical, simplistic, and non-invasive with accurate results. Additionally, the use of this stain can assist in determining the most appropriate biopsy sites and to surgically delineate margins or malignant areas. One of the disadvantages of using this stain includes the risk of obtaining false negative reactions and the patient is not followed up adequately, resulting in a tissue biopsy.
In addition to the brush biopsy, your doctor may perform one or more of the following tests:
• X-rays to see if cancer cells have spread to the jaw, chest, or lungs.
• CT scan to reveal any tumors in your mouth, throat, neck, lungs, or elsewhere in your body.
• PET scan to determine if the cancer has traveled to lymph nodes or other organs.
• MRI scan to show a more accurate image of the head and neck, and determine the extent or stage of the cancer.
• Endoscopy to examine the nasal passages, sinuses, inner throat, windpipe, and trachea.
Cancer Genetics
Cells of the mouth divide quickly during development in the womb and throughout infancy. This rapid division slows down greatly as an individual ages so that it is just sufficient to replace cells that are dead or injured. The division of cells in the mouth and elsewhere is under very tight control, regulated by genes in the cells. When this control is lost, the cells begin to divide in a haphazard way, and grow to form a swelling of abnormal cells--a tumor. A benign tumor grows only within its local area, not spreading to distant organs. It is not cancer. A malignant tumor can spread to another area of the body and it is a cancer. Cancers of the mouth tend to grow to large sizes locally before they metastasize, but any cancer can spread at any time.
Proto-oncogenes
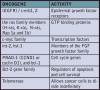
Scientists now understand that multiple mutations in specific classes of genes contribute to cancer of the head and neck. The two classes most fully characterized to date are proto-oncogenes and tumor suppressor genes. Proto-oncogenes code proteins that stimulate cell division. Altered forms of these genes called oncogenes can cause stimulatory proteins to be overactive, resulting in a cell dividing more rapidly than normal.
The oncogenes identified thus far as possible participants in oral cancer are shown in Table 2.
Tumor Suppressor Cells
Tumor suppressor genes code proteins that inhibit cell division. When these genes mutate, the corresponding protein is no longer produced correctly (in form, amount, or at time needed) and cell division may occur when it should not. Inactivated tumor suppressor genes that are suspected in oral cancer include Rb, p16 (MTS1 or CDKN2), E-cadherin, doc-1 and p53. E-cadherin is responsible for cell-cell adhesion and differentiation while doc-1 is induced by TNF-α and results in the inhibition of apoptosis and is deleted in oral cancer. P53 is already involved in approximately 60% of all human cancers.
P53
There has been much interest in p53 since it was discovered that the gene can stop tumor formation when functioning properly. p53 is located on the short arm of chromosome 17. It works by recognizing damage to a cell's DNA and preventing continued cell growth and division until the damage is repaired. If unable to repair, p53 causes the cell to experience apoptosis. p53 is also the gatekeeper of genome transcription and regulator of the angiogenesis inhibitor thrombospondin-1.
Appendix A (Table 3) lists the oral cancer genes and unique genes found in the Cancer Genome Anatomy Project so far. Appendix B (Table 4) lists the genes involved in oral cancer genetics that have been reported in literature to be up-regulated. Oral cancer results in many chromosomal changes. Chromosomal changes include recurrent chromosomal loss of 9, 13, 18, and 4; chromosomal deletions of 3p, 7q, 8p, 11q, and 17p; and chromosomal breakpoints in the centromeric regions of 1, 3, 14, and 15 on bands 1p22, 11q13, and 19p13. With ongoing research, more will be learned about the genetics of oral cancers.
Cancer Development
The stages of oral cancer development are similar to the stages of many other cancers (Table 5 and Table 6). Once an actual diagnosis has been made, the degree of development and invasiveness is also determined. This determination aids in the treatment planning and prognosis for the patient. Although it is true that individuals who are diagnosed with cancer in advanced stages have a poorer prognosis of both cure and survival; it does not automatically mean that these individuals will have a poorer outcome.
Metastasis
Although the spread of oral cancer to regional neck lymph nodes is common and indicates an advanced tumor, metastases to other organ systems below the clavicle are rare. If a tumor should metastasize below the clavicle, the lung is the most common site. Metastases from oral cancers occur primarily through the lymphatic system, while distant metastases are hematogenous. The ability of malignant cells to metastasize varies among patients and depends on certain cell surface molecules and extracellular matrix interactions.
In order for a tumor to metastasize, the malignant cells must first break free from adjacent cells and the extracellular matrix. Cells are normally held close together with cell-cell adhesion molecules, allowing for interactions between the proteins on the cell surface. Cadherins, a type of adhesion cell molecule, is found to be partly or entirely missing in malignant cells. Without these molecules, the tumor is free to detach and travel to another site. Research has shown that saliva provides a good environment for metastasis. Hyaluronic acid, a molecule that binds to surfaces of the cell, is found in abundance in saliva and makes it easier for cells to move around, aiding in the escape from cell adhesion.
Another type of molecule called an integrin is needed to anchor the normal cell to the extracellular matrix. Without being anchored, a normal cell cannot reproduce and will eventually die. A nuclear protein called E-CDK2 regulates the cell cycle. Cancer cells do not need to be anchored to the extracellular matrix in order to reproduce. The cell cycle regulator E-CDK2 remains active and allows for continued proliferation. The exact reason for this integrin remaining active in malignant cells is uncertain, although researchers feel that oncogenes may be a key factor. Once a cancer cell has detached from both its neighbors and the extracellular matrix, it must find a way into the blood or lymphatic system for transport. In order to enter the bloodstream, the malignant cell must penetrate the basement membrane of the epithelial cells by releasing enzymes called metalloproteinases, which dissolve the basement membranes and extracellular matrices. Only certain cells of the malignant tumor are able to metastasize, and not all cancer cells have metastatic properties.
Treatment Options
Treatment for oral cancer is different than other cancers. It is critical to get prompt diagnosis and proper treatment for a mouth cancer condition. It can mean the difference between life and death. The choice for cancer treatment is dependent upon the type and the stage of cancer. It may include surgery, chemotherapy, radiation, hormones, and/or immunotherapy. Some cancers respond to a single mode of treatment, whereas others require multi-modality treatment strategies.
The ultimate goal of the treatment is to remove totally or destroy the malignant cells from the body. Unfortunately, treatments available today are not able to target only the cancer cells, and normal healthy cells must sometimes be destroyed in the process of treating the cancer. This can result in significant psychological stress and physical morbidity or death. The goals of multi-disciplinary care are to cure the disease and prevent and assuage the side effects of treatment. Treatment does not stop at ridding the individual of cancer, but goes on to include the management of side effects of the treatment, and plan for reconstruction and rehabilitation. The dental profession plays a major role in the management of oral complications associated with cancer therapy and the prevention in some of the systemic problems that may occur.
Choice of treatment for oral cancer is dependent on the stage of the disease at the time of diagnosis. A small lesion of less than 1 cm may only require surgery or radiation therapy. Larger cancers, especially those that have spread to the lymph nodes in the neck, generally require surgery and radiation. Chemotherapy is not a curative treatment for oral squamous cell cancer, but it may be used as an adjunct before surgery or radiation therapy to reduce the size of the tumor, or as a palliative treatment for recurrent and advanced tumors.
Surgery
Surgery is preferred as the main treatment when oral cancer is not sensitive to radiation therapy, when lymph nodes, salivary glands, or bone are involved, or when there is a recurrence of a tumor in a site that has already received a therapeutic dose of radiation. The disadvantage of surgery is the sacrifice of important, functional oral structures.
Acute physical complications after head and neck surgery may include any of the following: airway obstruction, fistula formation, necrosis in the surgical site, impairment of the senses (hearing, vision, smell, taste), impairment of swallowing and speech, and compromised nutritional status. Long-term complications include speech impairment, malnutrition from the inability to swallow foods, drooling, malocclusion, temporomandibular disorders, facial deformity, and chronic pain in the shoulder muscles.
There may be consequential psychological problems associated with surgery because the results of the treatment are often visible and can be devastating and humiliating. Physical impairments cannot be completely disguised by clothing, cosmetics, or prostheses. Consequently, some individuals experience depression, withdrawal, anger, and/or stigmatization. Surgical procedures for oral cancer result in long-term disability. These problems may be short-term if reconstructive surgery by an oral surgeon and rehabilitation are possible.
Chemotherapy
Chemotherapy is often used as an adjunct to another treatment option. Chemotherapy is the use of certain chemicals to interfere with cancer cell proliferation, eventually leading to apoptosis. There are six classes of chemotherapy drugs and each one affects the cell's chemistry differently, depending on location of the cancer and the stage in which the cells are in the cell cycle (Table 7).
Depending on the drug chosen, current chemotherapy will affect malignant cells in one of three ways:
| 1. | Damage to the DNA of the cancer cells so they can no longer proliferate. This is done by alter- ing the DNA structure in the nucleus of the cell, thus preventing replication. |
| 2. | During the S phase of the cell cycle, the chemo agent inhibits the synthesis of new DNA strands so that no replication is possible. This occurs when the drugs block the formation of nucleotides that are necessary for new DNA to be produced. |
| 3. | Stop the mitotic processes of the cell so that the cancer cell cannot divide into two cells. The formation of the mitotic spindles is necessary to move the original DNA to the opposite sides of the cell so the cell can divide into two daughter cells. |
Chemotherapy is an effective way of killing malignant cells, but in the course of treatment normal cells are also killed. Once treatment has been completed, the non-cancerous cells will return to their normal function or be replaced by new healthy cells.
There are many side effects associated with chemotherapy that can bring discomfort and inconvenience. Most side effects are manageable, and are treatable if they do become problematic. Some common side effects include hair loss, mouth sores, gastrointestinal problems (nausea, constipation, diarrhea, loss of appetite), low blood cell counts, sore throat, rashes, fatigue, and infertility (temporary or permanent). One very important side effect is the effect chemotherapy has on bone marrow and the maturing red and white blood cells and platelets found there. The strong drug agents affect the quickly dividing marrow cells in the same way as the malignant cells. Decreased output of healthy red blood cells can lead to anemia, while a decreased output of white blood cells and platelets can lead to a compromised immune system and clotting and healing ability.
IMRT Radiation
IMRT is a state of the art cancer treatment that delivers high doses of radiation directly to the cancer cells in a precise, targeted way, while sparing more of the surrounding healthy tissue. This has important advantages in oral cancer as it allows the beam to hit the target area while missing the surrounding structures such as the salivary glands and sinuses. IMRT uses computer-generated images for planning and then delivers a tightly focused beam to the cancerous tumor. Clinicians "paint" the tumor with the radiation beam, which conforms closely to the shape of the tumor. IMRT can be used to treat tumors that in the past have been considered untreatable given their close proximity to critical organs and structures. A powerful computer program optimizes a treatment plan based on tumor size, shape, location, and dose requirements from the physician. A medical linear accelerator, equipped with a special device called a multi-leaf collimator that shapes the radiation beam, delivers the radiation according to instructions. The equipment can be rotated to access the tumor from the most favorable vantage point. IMRT is currently being used to treat other tumors of the head and neck, brain, liver, lung, pancreas, prostate, and uterus.
Radiation Therapy
Radiation therapy, also known as radiotherapy, is used in treating localized solid tumors, such as those in the head and neck. Before starting treatment, a CAT scan is done and measurements and markings are made on it in preparation of treating the tumor. A porous mesh mask is made for the patient to immobilize the head during treatment, and to prevent irradiation of healthy areas. The total dose of radiation is prescribed by a radiation oncologist and broken down into smaller doses to be given over a period of days. Most patients tolerate these smaller doses without an adverse effect on the maximum benefit. The treatment course is usually 2-8 weeks, allowing for normal tissues to repair after each exposure and minimizing permanent injury. Research is being conducted to increase the effectiveness of current techniques.
Recent radiotherapy research has focused on the use of radiolabeled antibodies to deliver doses of radiation directly to the cancer site. Some tumor cells contain specific antigens that trigger the body's immune system so that it produces tumor specific antibodies. Benefits of this approach include minimizing the risk to healthy tissues. Safe, but effective dosages and identification of appropriate radioactive substances are still being worked out. Side effects of radiation therapy include irritation of the tissue in the treatment area, xerostomia, infection, radiation-induced caries, trismus, alteration of taste, possible hair loss, and fatigue.
Osteoradionecrosis is a condition that affects bone healing and can occur in individuals who receive high doses of radiation during cancer therapy. This can occur after dental surgery or extraction of teeth. High doses of radiation can decrease the bone's blood supply, resulting in the decrease of oxygen to the area and eventual necrosis of bone tissue. The mandible is the most often affected area. Symptoms of osteoradionecrosis may occur months to years after radiation therapy with common symptoms including mouth pain, swelling of the jaw, and trismus. Treatment of osteoradionecrosis can vary from antibiotic therapy, local debridement, and in more severe cases removal of affected bone.
Prognosis
Prognosis is highly variable and dependent upon the stage and the location of the disease when first diagnosed. The more advanced stages will result in a lower survival rate. The survival rates for carcinomas of the base of the tongue (distal to the circumvallate papillae) are very low compared with those carcinomas on the oral portion of the tongue. The American Cancer Society reported in 2013 that the 5-year survival rate for stage I cancer of the lip is 96% whereas the 5-year survival rate for stage I cancer of the oropharynx and tonsil is only 56%. The public often does not comprehend the severity of oral cancer, perhaps because it associates diseases in the mouth with dental care that is not life threatening.
The 5-year survival rate refers to the percentage of patients who live at least 5 years after their cancer is diagnosed even though many of these people go on to live much longer than 5 years. Five-year relative survival rates, such as the numbers in Table 8, assume that some people will die of other causes and compares the observed survival of people with cancer with that expected for people without cancer. This is a more accurate way to describe the impact that a particular type and stage of cancer may have on survival. To figure out 5-year survival rates, doctors have to look at patients who were treated at least 5 years ago. Improvements in treatment since then may result in a more favorable outlook for people now being diagnosed with these same cancers. Survival rates are often based on earlier outcomes of large numbers of people who had the particular disease, but they cannot predict what will happen in any individual’s case. Many other factors may affect a person’s outlook, such as the patient’s age and health, the treatment received, positive outlook, and how well the cancer responds to treatment.
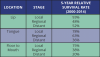
The survival statistics come from the National Cancer Institute’s SEER program. They are based on large numbers of patients diagnosed with particular cancers between 2000 and 2014. SEER doesn’t provide recent statistics by the typical AJCC stage as referenced earlier. Instead, cancers are divided into the summary stages:
• Local: The cancer is only in the area where it started. This includes stages I and II, as well as some stage III cancers that haven’t spread to any lymph nodes.
• Regional: The cancer has spread to nearby tissues and/or lymph nodes. This includes some stage III cancers, as well as stage IV cancers that haven’t spread to distant sites.
• Distant: The cancer has spread to distant sites.
These statistics are based on the stage of the cancer when it was first diagnosed for the patient. The statistics do not apply to cancers that have come back or spread.
For cancers of the oropharynx and tonsil, the relative 5-year survival rate was 66%, but survival by stage is not available, while cancers of the gums and other parts of the mouth, the relative survival were 60%, but survival by stage is not available.
Research
Ongoing research is being done in several areas of oral cancer at many university hospitals, medical centers and other institutions nationwide. Each year more is learned about the deadly disease. Current research is being done in the areas of DNA changes, gene therapy, new chemotherapy agents and radiotherapy methods, tumor growth factors, and vaccines.
DNA Changes
Work is being done with the p53 gene and HPV DNA. Tests are being formed to detect p53 alterations, allowing for early detection of oral and oropharyngeal tumors. Tests are also being conducted for detection of HPV DNA in cells for earlier diagnosis of other forms of cancer.
Gene Therapy
Research in reversing DNA changes in oral cancer as a treatment is being conducted. Clinical trials involving p53 and its replacement with a normal gene to restore normal function to the cells currently are being conducted. Gene therapies to disrupt HPV replication also are being developed. Another type of therapy involves new genes being added to cancer genes to make them more susceptible to a particular type of drug in treating the particular type of cancer.
New Chemotherapy and Radiotherapy Methods
New drugs are being developed that are more effective against advanced oral cancer. Intraarterial chemotherapy (injection of drugs into the arteries feeding the cancer) is being tested in conjunction with radiation therapy. Advances in intralesional chemotherapy are being made. New methods are also being developed in radiotherapy that will reduce side effects and tissue/organ destruction.
Tumor Growth Factors
New drugs that recognize cells with too many Epithelial Growth Factors receptors are now being tested in clinical trials. These drugs also may boost the patient's immune system.
Vaccines
Vaccines are being investigated as a way to treat cancer patients by helping to boost their immune systems to recognize and attack cancer cells. Vaccines against HPV have been developed and licensed for use as of June 2006. The vaccine, Gardasil®, is made up of proteins from the outer coat of the virus and does not contain any infectious material. Gardasil® protects against four types of HPV, attributing to 75% of the cervical cancer cases and 90% of genital warts. This vaccine, recently approved for use in females and males aged 9-26 years, is given through a series of three shots over a period of six to twelve months. Studies have found Gardasil® and Gardasil®9 to be almost 100% effective in preventing diseases caused by the four types of HPV (6, 11, 16 and 18) covered by the vaccine, but less effective in individuals who had already been exposed. This vaccine is not used in treating existing HPV infections, genital warts, precancers or cancers. Gardasil® is designed to protect against two of approximately 23 known high-risk HPV types. Gardasil®9 is designed to protect against seven high-risk HPV types. Both are designed to protect against HPV-16 and HPV-18.
Dental Considerations for the Diagnosed Patient
It can be challenging caring for a patient who is undergoing cancer treatments or will be in the near future. Patient education is an essential part of the pretreatment assessment and should include a discussion of possible oral complications.
Prior to Treatment
A thorough exam of the hard and soft tissues:
| • | Reduces the risk and severity of oral complications |
| • | Allows for immediate identification and treatment of existing infections and other problems |
| • | Increases the probability that the patient will successfully complete the prescribed course of treatment |
| • | Reduces the likelihood of oral pain |
| • | Minimizes oral infections that could lead to potentially fatal systemic infections |
| • | Minimizes complications that compromise nutrition |
| • | Preserves or improves oral health by improving the quality of life and decreasing the cost of care in the future |
| • | Establishes a baseline for comparison post treatment |
The following measures should also be taken into consideration:
| • | Identify and treat existing infections, carious lesions, and other compromised teeth |
| • | Stabilize or eliminate potential sites of infection |
| • | In adults, extract teeth that may pose a future problem or are non-restorable to prevent later extraction-induced osteonecrosis |
| • | In children, consider extracting highly mobile primary teeth and teeth that are expected to exfoliate during the course of treatment |
| • | Perform oral surgery at least 2 weeks before any radiation therapy begins |
| • | Oral surgery should be performed at least 7 to 10 days before the patient receives myelosuppressive chemotherapy |
| • | Remove orthodontic bands and brackets if highly stomatoxic chemotherapy is planned or if the appliances will be in the radiation field |
| • | Prescribe an individualized oral hygiene regime to minimize oral complications |
| • | Patients undergoing head and neck radiation therapy should be instructed in the use of supplemental fluoride |
| • | If a removable prosthetic appliance is worn, make sure it is well adapted to the tissues and that the patient is able to wear and clean it daily; Instruct the patient to leave the appliance out at night, allowing the tissues to breathe |
Post Treatment
Continued patient education is needed during and after cancer treatment. Instruction in adequate nutrition and avoiding alcohol and tobacco can prevent or decrease oral complications. Detailed instructions should be provided for the patient on specific oral health care practices such as how and when to brush and floss, how to recognize signs of oral complications, and other instructions appropriate for the individual. Instructions may include:
| • | Brush teeth, gums, and tongue gently with an extra-soft toothbrush and fluoride toothpaste after every meal and before bed; Bristles may be softened on the brush with warm water |
| • | Floss teeth gently every day; It is recommended that if gums are sore or bleeding, to avoid those areas and floss the other teeth |
| • | Avoid alcohol-containing mouth rinses, spicy or acidic foods, toothpicks, tobacco products, and alcoholic beverages |
| • | Avoid candy, gum, and soda unless they are sugar-free |
| • | Rinse the mouth with a baking soda-salt solution followed by a plain water rinse several times a day (Use 1/4 teaspoon of baking soda and 1/8 teaspoon of salt in 8 ounces of warm water) |
| • | Exercise the jaw muscles three times a day to prevent and treat jaw stiffness; Open as far as possible without causing pain and close the mouth; repeat 20 times |
| • | Follow instructions for using fluoride gel |
| • | Keep all appointments recommended by the dentist. |
Supplemental Fluoride
Supplemental fluoride is often prescribed during cancer treatment as a fluoride gel delivered via custom trays. Fluoride rinses are not adequate to prevent demineralization. Several days prior to the start of radiation therapy, a daily regimen of a 5-minute application using 1.1% neutral pH sodium fluoride gel or 0.4% stannous fluoride should be started. Whether stannous or sodium fluoride is used, the patient should always use unflavored fluoride. Flavored fluorides can be irritating to already inflamed and possibly painful oral tissues. For patients with porcelain crowns, composite, or glass ionomer restorations, a neutral pH fluoride should be used to prevent damage to the restorations.
When using a tray to deliver the fluoride application, the following instructions should be given to the patient (Figure 11):
| 1. | Place a thin ribbon of fluoride in each tray |
| 2. | Place the trays on the teeth and leave in place for 5 minutes; If the gel overflows the tray, too much is being used and the amount should be reduced |
| 3. | After 5 minutes, remove the trays and spit out any excess gel; Do not rinse |
| 4. | Do not eat or drink for 30 minutes |
| 5. | Clean the trays with a toothbrush and allow to air dry; Trays can be cleaned weekly in a denture cleaner following manufacturer's instructions |
For those patients reluctant to use a fluoride tray, a high-potency fluoride gel should be brushed on the teeth following daily brushing and flossing. Either 1.1% neutral pH sodium or 0.4% stannous fluoride gel is recommended, based on the type of dental restorations present in the mouth.
When using the brush-on method of application the following instructions should be given to the patient (Figure 12):
| 1. | After brushing with fluoride toothpaste, rinse as usual |
| 2. | Place a thin ribbon of fluoride gel on the toothbrush |
| 3. | Brush for 2 to 3 minutes by the clock or timer |
| 4. | Spit out any excess gel at the end of the timed period; Do not rinse. |
| 5. | Do not eat or drink for 30 minutes |
| 6. | Rinse toothbrush well and allow to air dry |
Patients with radiation-induced salivary gland dysfunction must continue lifelong daily fluoride application to help preserve the dentition.
Once all complications of chemotherapy have been resolved, patients may be able to resume their normal dental care schedules. However, if the patient's immunity continues to be compromised, the patient's hematologic status needs to be determined before any dental treatment or surgery is initiated. This is particularly important to remember for those patients who have undergone blood/platelet transfusion therapies and marrow transplantation.
Patients who have completed radiation therapy to the head and neck and whose oral complications have decreased should be evaluates every 4 to 8 weeks for the first six months. Time intervals can be changed depending upon the patient's dental needs. It is, however, important to remember that oral complications may continue or appear long after radiation therapy has ended.
Summary
Continued research is needed in the fight against all cancers. Basic research has already paid off in many ways. Researchers know which specific classes of genes are involved in cancer development, that environmental factors can trigger genetic mutations, and that cancer cells can become out of control and proliferate without restraint. The general populace needs further education about changing lifestyle factors. Quitting all forms of tobacco use, stopping excessive use of alcohol, making sure dental appliances fit appropriately, and eating a diet rich in fruits and vegetables are all things people can do now to reduce their risk of developing the disease. Age appropriate HPV vaccinations can also help reduce oral cancer frequency.
Historically the death rate associated with this cancer is particularly high not because it is hard to discover or diagnose, but due to the cancer being routinely discovered late in its development. Today, in 2017, that statement is still true, as there is not a comprehensive program in the United States to opportunistically screen for the disease, and without that, late stage discovery is more common. Another obstacle to early discovery and resulting better outcomes is the advent of a virus, HPV-16, contributing more to the incidence rate of oral cancers, particularly in the posterior part of the mouth (the oropharynx, the tonsils, the base of tongue areas) which many times does not produce visible lesions or discolorations that have historically been the early warning signs of the disease process. Medical and dental professionals can save more lives by consistently performing simple head and neck exams on all patients, and by discussing with them the early warning signs of oral cancer.
Head and neck exams should become the standard protocol for each new patient entering a practice, as well as for patients returning for routine care. The importance of a tobacco-free lifestyle should be addressed with patients who use. Various modalities should be incorporated into their care, whether it is prescribing a nicotine patch or a referral to a tobacco cessation clinic at a dental school or medical center.
Glossary
adenopathy - any disease of the glands, especially of the lymphatic glands. It is also referred to as lymphadenopathy.
allele - one of two or more substitute forms of a gene that happen by mutation and are found at the same place on a chromosome. Most genes have a dominant allele and a recessive allele.
angiogenesis - the process that stimulates the formation of blood vessels. Tumor angiogenesis is the growth of new blood vessels that the tumor needs to grow through the release of chemicals by the tumor and host cells near the tumor.
apoptosis - a genetically directed process of cell self-destruction that is marked by the fragmentation of nuclear DNA, is activated either by the presence of a stimulus or removal of a suppressing agent or stimulus, is a normal physiological process eliminating DNA-damaged, unnecessary, or unwanted cells, and when halted (as by genetic mutation) may result in uncontrolled cell growth and tumor formation — called also cell suicide or programmed cell death.
asymptomatic - presenting without symptoms.
basement membrane - a thin extracellular supporting layer that separates a layer of epithelial cells from the primary lamina propria and is composed of the basal lamina and reticular lamina.
basil lamina - a thin extracellular layer composed chiefly of collagen, proteoglycans, and glycoproteins that lies next to the basal surface of epithelial cells that separates these cells from underlying or surrounding connective tissue or adjacent cells.
benign - not recurrent or progressive; non malignant or harmless.
carcinogenic - causing substance or agent causing cancer.
carcinoma - a new growth or malignant tumor that occurs in epithelial tissue; etiology is unknown.
centromere - region of the chromosome that connects the chromatids during cell division.
chromatid - one of two potential chromosomes formed by DNA replication.
chronicity - failure to heal therefore lasting a long time.
cell suicide - another word for apoptosis.
clone - group of cells descended from a single cell and each are identical in nature.
collagen - any of a group of fibrous proteins that occur in vertebrates as the main component of connective tissue fibrils and in bones and yield gelatin.
conjugated - formed by the union of two compounds.
differentiation - the acquiring of individual characteristics.
DNA - deoxyribonucleic acid; carrier of genetic information in plants and animals.
down-regulate - to inhibit or suppress the normal response of a gene due to a decrease in the number of receptors on the cell surface.
dysplastic - abnormal growth or development of tissue.
epithelial - a type of tissue cell that forms the outer layer of skin and lines the inner cavities of the body; arranged in one to few layers and lacking blood vessels.
erythema - a red area of variable shape and size reflecting inflammation, thinness, irregularity of the epithelium and lack of keratinization.
erythroplakias - red lesions of the mucous membranes that cannot be attributed to any other pathology.
etiology - the cause of the disease.
extracellular matrix - is a collection of molecules outside the cell secreted by cells that provides structural and biochemical support to the surrounding cells.
fibrils - small filaments or fibers.
fixation - a non-mobile lesion occurring as a result of abnormally dividing cells invading to deeper areas and onto muscle and bone.
free radicals - molecules that have split and now have an odd number of electrons; often repaired with antioxidants found in fruits and vegetables.
glycoproteins - a protein in which the non protein group is a carbohydrate.
hematogenous - originating in the blood.
heterozygosity - possessing different alleles.
immunosuppression - prevention of the activation of the immune responses by drugs or disease.
induration - hardness, primarily as a result of an increase in the number of epithelial cells from an inflammatory reaction that has spread into the surrounding tissue.
innocuous - producing no injury; harmless.
integrin - the receptor on cell surfaces that links with proteins and chemical mediators for cell-to-cell communication.
Kaposis's Sarcoma - a vascular malignancy that is often first apparent in the skin or mucous membranes; the most common AIDS-related tumor; etiology is unknown but thought to involve immunosuppression.
keratin - an extremely tough, but fibrous protein substance that may be hard or soft.
keratinization - the process of keratin formation.
lamina propria - a highly vascular layer of connective tissue under the basement membrane lining a layer of epithelium.
lateral borders of the tongue - sides of the tongue.
leukoplakia - a white patch on the mucosal surface that is abnormal.
library - a collection of cloned DNA fragments that are preserved in a proper cellular environment and that usually represent the genetic material of a particular organism or tissue.
localized - restricted to a limited area.
lymphadenopathy - disease process affecting the lymph nodes resulting in the hardening and enlargement of the nodes; sometimes referred to as adenopathy.
lymphoma - a usually malignant tumor of lymphoid tissues.
malignant - cancerous growths resisting treatment ultimately resulting in death of the organism.
melanoma - a malignant tumor of the skin containing dark pigment.
metabolites - byproducts of metabolism.
metalloproteinases - any protease enzyme whose catalytic mechanism involves a metal.
metastasis - movement of cancerous cells from one part of the body to another.
mutations - the changing of the structure of a gene, resulting in a variant form that may be transmitted to subsequent generations, caused by the alteration of single base units in DNA, or the deletion, insertion, or rearrangement of larger sections of genes or chromosomes.
myeloma - a tumor originating in the cells of the bone marrow.
neoplasm - a new and abnormal formation of tissue (tumor) that grows at the expense of the healthy organism.
oncogenes - genes that have the ability to induce cell malignancy.
oropharynx - the central portion of the pharynx lying between the soft palate and the upper portion of the epiglottis.
p53 - a protein produced by a tumor-suppressor that is believed to play an important role in the birth and death of cells.
pathology - the typical behavior of a disease.
programmed cell death - another word for apoptosis.
protease - a class of enzymes that break down amino acid proteins.
proteoglycan - any of a class of glycoproteins of high molecular weight that are found especially in the extracellular matrix of connective tissue.
pyrolysis - chemical change brought about by the action of heat.
proto-oncogenes - normal cell genes before they become oncogenes.
radiation induced caries - carious lesions that develop in reaction to head and neck radiation; during and following treatment, the patient experiences xerostomia, changes in salivary flow and perhaps difficulty in maintaining proper oral hygiene.
reticular lamina - a thin extracellular layer that sometimes lies below the basal lamina, is composed chiefly of collagenous fibers, and serves to anchor the basal lamina to underlying connective tissue.
squamous cell - a flat, scaly, epithelial cell.
stage - period in the course of a disease such as cancer; the higher the number of the stage, the more advanced the disease.
stomatoxic - toxicity to the oral cavity.
telomerase - an enzyme present in cancer cells that allows them to divide indefinitely.
transformation - changing of shape or form.
trismus - a contraction of the jaw muscles as a result of oral infection, trauma, or inflammation of the salivary glands.
tubulin - a protein present in cells.
tumor - a spontaneous new growth of tissue forming a mass that can be benign or malignant.
tumor suppressor genes - genes that suppress the growth of a tumor.
ulceration - loss of skin surface with a gray to yellow center surrounded by a red halo, resulting from the destruction of epithelial integrity owing to discrepancy in cell maturation, loss of intracellular attachments, and disruption of the basement membrane; formation of a sore (ulcer).
up-regulate - to increase or excite the normal response of a gene.
ventral surface of the tongue - under side of the tongue.
xerostomia - abnormal dryness of the mouth due to insufficient secretions as a result of medication, medical condition or radiation therapy.
References
http://www.medschool.lsuhsc.edu/genetics_center/louisiana/article_oralcavity2_p.htm - accessed July 30, 2017
http://oralcancerfoundation.org/ - accessed July 29, 2017
https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet - accessed July 30, 2017
Alfano, DMD, Ph.D, M. C., & Horowitz, Ph.D, A. M. (2001). Professional and community efforts to prevent morbidity and mortality from oral cancer. Journal of the American Dental Association JADA, 132(Novemeber), 24S-28S.
American Association Oral Maxillofacial Surgeons. (2007). Oral cancer. Retrieved from http://www.aaoms.org/oral_cancer.php
American Cancer Society. (2013). Cancer facts and figures. Retrieved from http://www.cancer.org/research/cancer-factsfigures/cancerfactsfigures/cancer-facts-figures-2013
American Dental Assistants Association. (2001). Early detection of oral cancer with brush biopsy. The Dental Assistant Journal, May- June,
American Dental Association. (2013, January). Statement on human papillomavirus and squamous cell cancers of the oropharynx. Retrieved from http://www.ada.org/1749.aspx
Bhoopathi, V., Kabani, S., & Mascarenhas, A. K. (2009). Low predictive value of the oral brush biopsy in detecting dysplastic oral lesions. Cancer, 115(5), 1036- 1040. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/cncr.24089/pdf
Cancer Care Inc. (2006). A team approach to treating head and neck cancer. Retrieved from http://oralcancer-foundation.org/treatment/pdf/CC_ team_brochure.pdf
Casiglia, DMD, J., & Woo, DMD, MMSc, S. B. (2001). A comprehensive review of oral cancer. Journal of the Academy of General Dentistry, January-February, 72-82.
CDC Centers for Disease Control and Prevention. (2012). Human papillomavirus-associated cancers- United States, 2004-2008. MMWR, 61(15), 258-261. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrht-ml/mm6115a2.htm
Centers for Disease Control and Prevention. (2013, November 13). Human papillomavirus (hpv) and oropharyngeal cancer - fact sheet. Retrieved from http://www.cdc.gov/std/hpv/stdFact-HPVandoralcancer.htm
Drinnan MD,DDS, A. (2000). Screening for oral cancer and precancer- a valuable new technique. Journal of the Academy of General Dentistry, November-December, 656-660.
Fred Hutchinson Cancer Research Center Survivorship Program. (n.d.). Retrieved from http://www.fhcrc.org/patient/support/survivorship/resources/Healthlink.Osteoradionecrosis.pdf
Grayzel, E. (N.D.). Six step screening: The best practice in oral care. Retrieved from http://www.sixstepscreening.org/
Horowitz, Ph.D, A. M., & Alfano, DMD, Ph.D, M. C. (2001). Performing a death-defying act. Journal of the American Dental Association JADA, 132(November)
LED Dental (2009). Accessed January 25, 2014 from http://www.leddental.com/velscope-technology/tissue-fluorescence/
Lopez, DDS, Ph.D, M. A., Pazoki, MD, DDS, A. E., & Ord, DDS, MD, FRCS, FACS, R. A. (2000). Proliferative verru- cous leukoplakia: A case report. Journal of the Academy of General Dentistry, November-December, 708-710.
Marder, DDS, M. Z. (2001). What are the diagnostic protocols for oral cancer screenings?. Journal of the American Dental Association JADA, 132(January), 83-84.
Massler Jr, DDS, MEd, ABGD, MAGD, C. F. (2000). Preventing and treating the oral complications of cancer therapy. Journal of the Academy of General Dentistry, November- December, 652-654.
National Institute of Dental and Craniofacial Research. (November, 2006). Oral cancer 5-year survival rates by race, gender, and stage of diagnosis. Retrieved from http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/OralCancer/OralCancer5YearSurvivalRates.htm
Ord, MD, DDS, FRCS, FACS, MS, R. A., & Blanchaert Jr, MD, DDS, R. H. (2001). Current management of oral cancer: A multidisciplinary approach. Journal of the American Dental Association JADA, 132(November), 19S-22S.
Silverman Jr, MA, DDS, S. (2001). Demographics and occurrence of oral and pharyngeal cancers: The outcomes, the trends, the challenge. Journal of the American Dental Association JADA, 132(November), 7S-10S.
Stevenson, M. M. (2013, May 30). Oral cavity and laryngeal cancer staging. Retrieved from http://emedicine.medscape.com/article/2048034-overview
Sciubba, DMD, Ph.D, J. J. (2001). Oral cancer and its detection: History taking and the diagnostic phase of management. Journal of the American Dental Association JADA, 132(November), 12S-17S.
Tomar, DMD, Ph.D, S. L. (2001). Dentistry's role in tobacco control. Journal of the American Dental Association JADA, 132(November), 30S-34S.
What we know.. about oral cancer. (n.d.). Retrieved from http://www.nidr.nih.gov/Spectrum/NIDCR3/3grasec3.htm
To be or not to be… cell suicide as cancer therapy. (n.d.). Retrieved from http://nidr.nih.gov/Spectrum/NIDCR3/3grasec10.htm
Zila, Inc. (2010). Accessed January 25, 2014 from http://www.zila.com/vizilite/
(2001). Oral cancer: How to protect yourself. Journal of the American Dental Association JADA, 132(November), 48S.
(2001). Perform a death-defying act: The 90 second oral cancer examination. Journal of the American Dental Association JADA, 132 (November), 36S-40S.
About the Author
Natalie Kaweckyj, LDARF, CDA, CDPMA, COA, COMSA, CPFDA, CRFDA, MADAA, BA
Natalie Kaweckyj currently resides in Minneapolis, MN, where she is a clinician and part of the management team for a nonprofit pediatric dental clinic. She is a Licensed Dental Assistant in Restorative Functions (LDARF), Certified Dental Assistant (CDA), Certified Dental Practice Management Administrator (CDPMA), Certified Orthodontic Assistant (COA), Certified Oral & Maxillofacial Surgery Assistant (COMSA), Certified Preventive Functions Dental Assistant (CPFDA), Certified Restorative Functions Dental Assistant (CRFDA) and a Master of the American Dental Assistants Association. She holds several expanded function certificates, including the administration of nitrous oxide/oxygen analgesia. Ms. Kaweckyj graduated from the American Dental Association-accredited dental assisting program at ConCorde Career Institute and has received a Bachelor of Arts in Biology and Psychology from Metropolitan State University. She is currently writing her dissertation for her master’s in Public Health. She has worked clinically, administratively and academically. Ms. Kaweckyj is currently serving as on several ADAA Councils after having served on the ADAA Board of Trustees 2002 – 2012, and 2016 – 2019 She served as ADAA President in 2010-2011 and 2017 – 2018. She is the current Business Secretary and legislative chairman for the Minnesota Dental Assistants Association (MnDAA) and a three time past president of MnDAA. She also is a Past President of the Minnesota Educators of Dental Assistants (MEDA). In addition to her association duties, Natalie is very involved with the Minnesota state board of dentistry as well as with state legislature in the expansion of the dental assisting profession. She is a freelance writer and lecturer and is always working on some project. She has authored many other courses and webinars for the ADAA.